How to better manage and treat diabetes complications
The Innovative Health Initiative (IHI) is organizing an online webinar on February 4th, 2025 in conversation with BEAt DKD and HypoResolve.
This 45-minute online event will discuss the work of Beat-DKD and HypoResolve to address diabetes complications within the scope of public-private partnerships. The goal of this event is to highlight the projects’ achievements to a general audience, through a lively discussion amongst the panel. The discussions will revolve around the projects’ impact and the potential effect on the lives of patients.
You can read more about the event and the speakers, as well as register to participate at:
In conversation with... Beat-DKD and Hypo-Resolve | IHI Innovative Health Initiative
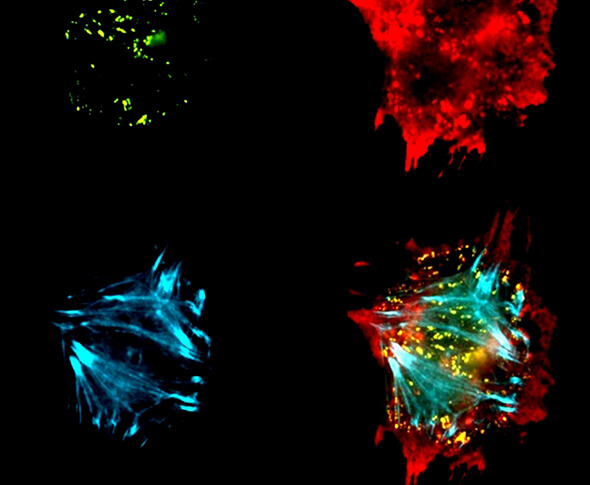
The image shows the four key cell types that were studied (left). To the right are podocyte cells in vitro.
Profiling of insulin-resistant kidney models and human biopsies reveals common and cell-type-specific mechanisms underpinning Diabetic Kidney Disease
Diabetic kidney disease (DKD) is the leading cause of end stage kidney failure worldwide, of which cellular insulin resistance is a major driver. In a study performed within the BEAt-DKD consortium, researchers studied key human kidney cell types implicated in DKD (podocytes, glomerular endothelial, mesangial and proximal tubular cells) in insulin sensitive and resistant conditions and performed simultaneous transcriptomics and proteomics for integrated analysis. The data was then further compared with bulk- and single-cell transcriptomic kidney biopsy data from early- and advanced-stage DKD patient cohorts. The authors identified several consistent changes (individual genes, proteins, and molecular pathways) occurring across all insulin-resistant kidney cell types, together with cell-line-specific changes occurring in response to insulin resistance, which are replicated in DKD biopsies. This study provides a rich data resource to direct future studies in elucidating underlying kidney signalling pathways and potential therapeutic targets in DKD. Link to the paper Profiling of insulin-resistant kidney models and human biopsies reveals common and cell-type-specific mechanisms underpinning Diabetic Kidney Disease Published in: Nature communications
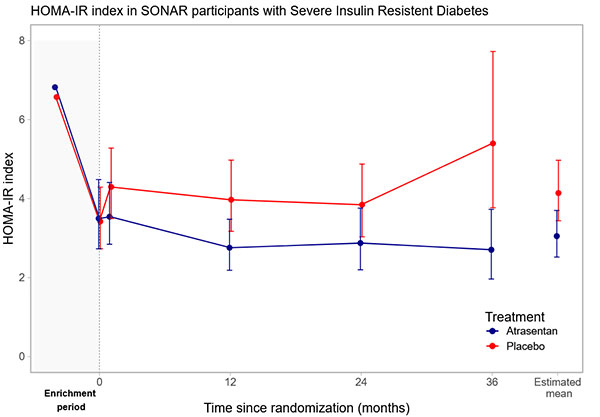
Figure shows the change in HOMA-IR for participants with the severe insulin resistance diabetes (SIRD) phenotype.
The effect of the endothelin receptor antagonist atrasentan on insulin resistance in phenotypic clusters of patients with type 2 diabetes and chronic kidney disease (CKD)
Earlier studies have demonstrated that patients with type 2 diabetes and CKD characterized by high levels of insulin resistance are at particularly high risk for progression of CKD towards kidney failure, necessitating dialysis and/or kidney transplantation. Endothelin receptor antagonists (ERA) are a class of drugs investigated for the treatment of CKD. An analysis of 931 patients with type 2 diabetes and CKD who participated in the SONAR clinical trial, found that the ERA atrasentan specifically improved insulin sensitivity among patients characterized by high levels of insulin resistance. This finding suggests that atrasentan may be a promising treatment option for patients with diabetes and CKD at high risk of developing kidney failure. While the drug also showed some positive effects on insulin sensitivity in other type 2 diabetes phenotypes, the impact was less pronounced. Further research is needed to explore whether the improved insulin sensitivity in response to atrasentan contributes to the long-term benefits of atrasentan and to determine its optimal use in clinical practice. This study provides novel insights into the potential therapeutic benefits of ERAs in a high-risk population. Link to the paper The effect of the endothelin receptor antagonist atrasentan on insulin resistance in phenotypic clusters of patients with type 2 diabetes and chronic kidney disease Published in: Diabetes, Obesity and Metabolism - Wiley Online Library

Prof. Heerspink was awarded the Donald W. Seldin Young Investigator Award during Kidney Week 2024.
Professor Hiddo Jan L. Heerspink Receives ASN-AHA Donald W. Seldin Young Investigator Award
The American Society of Nephrology (ASN) and the American Heart Association (AHA) awarded the Donald W. Seldin Young Investigator Award to Professor Hiddo Jan L. Heerspink, for his contributions to advancing clinical trial methodologies in chronic kidney disease (CKD) research.
Professor Heerspink has been vice-coordinator for BEAt-DKD and fundamental for driving activities in WP2. He currently coordinator for PRIME-CKD (Personalized drug Response: IMplementation and Evaluation in Chronic Kidney Disease; https://www.prime-ckd.com/). He has successfully led and currently leads numerous clinical trials, including trials of dapagliflozin in CKD (DAPA-CKD), finerenone in nondiabetic CKD (FIND-CKD), atrasentan in patients with immunoglobulin A nephropathy (ALIGN), and a zibotentan and dapagliflozin in treating CKD and high proteinuria (ZENITH-CKD).
On behalf of BEAt-DKD, we would like to congratulate Professor Heerspink on his award.
Professor Heerspink’s award lecture entitled “Innovations in Clinical Trial Design and Conduct for New Therapeutic Approaches in CKD” is available to watch here: Link to the video

New KDIGO guidelines
New KDIGO guidelines for the evaluation and management of chronic kidney disease (CKD) have been published. We are witnessing a transformative era in nephrology, with increasingly sophisticated diagnostic tools and advances in technology, with better integration of the latest evidence and expert consensus, all of which will hopefully help catalyze a more coordinated CKD care management worldwide, ultimately improving outcomes for people with CKD.
We are pleased to see KidneyIntelX™ developed by PRIME-CKD partner Renalytix, included as a biomarker-based risk assessment tool (page 87, Table 20) for CKD progression.
Link to the guidelines

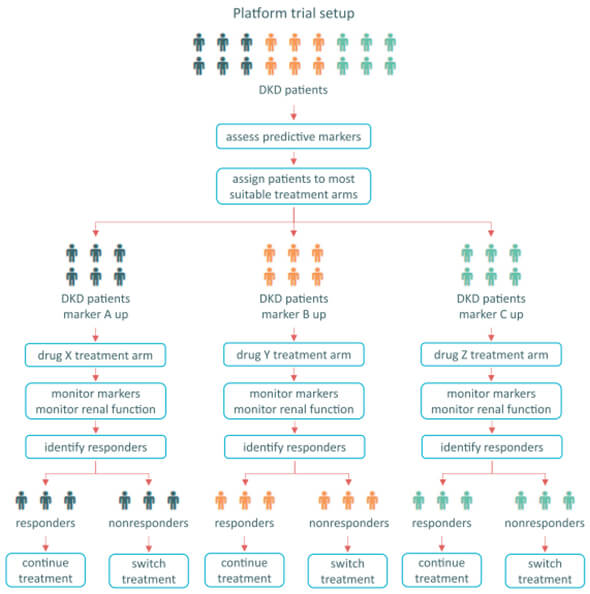
Regulatory support for the Multiple Parameter Drug Response score
The multiple parameter drug response score (PRE score) predicts the change in risk in reaching renal endpoints on the basis of the effects of a drug on multiple parameters involved in renal progression and protection. With this prediction, the PRE score intents to improve drug development and study design for registration trials in (diabetic) patients with chronic kidney disease. Various validation studies of the PRE score have been conducted, which have led the BEAt-DKD consortium members to reach out to the European Medicines Agency (EMA) for a so-called qualification opinion. This has resulted in a letter of support implying that the EMA supports the development effort towards intermediate and final validation of the PRE score as a tool for drug development in kidney diseases.
Link to the Letter of Support

Patient event
The iBEAt/BEAT-DKD study team is inviting its study participants to attend a patient event where the ideas behind the study will be discussed and iBEAt researchers can answer some of the questions that participants have been asking. During the event, a panel of experts will give short presentations on the various areas covered by the study, ending with a question-and-answer session.
The meeting will be held virtually or in person on Wednesday the 30th of August starting at 9 am and will last about 2.5 hours. All will be in English but live translation in Italian will be available.
Areas covered in this event:
- What is the BEAt-DKD consortium? Why is it interested in diabetic kidney disease?
- What is the iBEAt / BEAT-DKD study that you are participating in trying to achieve?
- Why do I need to be in the MRI scanner for so long?
- Why are you looking at the amount of fat and where it is, in my MRI images?
- Why are you collecting so much of my blood and urine? What happens to it?
- Why are participants in Bari, Italy also having a kidney biopsy? Why is this important?
- What is the microvascular study? Why are we interested in the smallest blood vessels?
- Why are big drug companies interested in being involved with the BEAt-DKD consortium?
The study team intends to record the event and a recording of each talk will be added to the BEAt-DKD website and will be available online after the event.
Link to agenda file
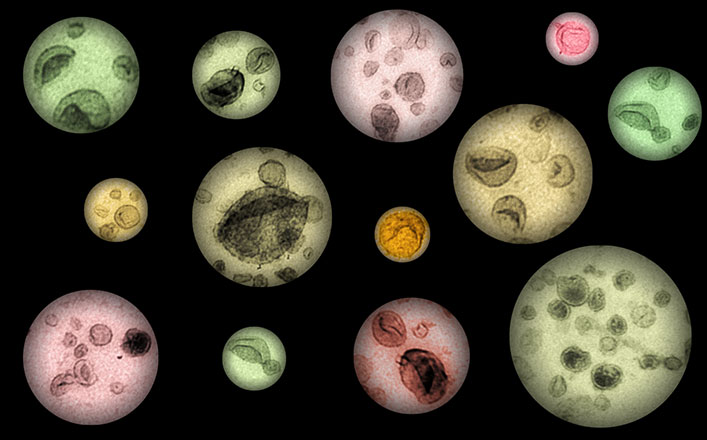
EV art by K. Barreiro: An electron micrograph of urinary extracellular vesicles that highlights extracellular vesicle subpopulations reflecting kidney derived vesicles carrying the stress score. (Image: Karina Barreiro)
The small extracellular vesicles in urine show transcriptomic stress signature in diabetic kidney disease
Genome-wide sequencing of mRNAs in urinary extracellular vesicles (EV) revealed a transcriptomic signature that associated with declining kidney function in diabetic kidney disease. The study included over 150 individuals with type 1 and type 2 diabetes from three different diabetes cohorts: FinnDiane, DIREVA, and iBEAt. The signature genes were involved in the management of oxidative and cellular stress and hence allowed calculation of an EV-based “stress score”. Significantly, the stress score proved to be higher in individuals showing an early declining trend of kidney function, but not yet albuminuria. The study additionally showed that EV and the stress signature in urine of the patients with diabetes are prominently coming from the kidney cortex and from proximal tubular cells. Thus, urine EV provide a potential “liquid kidney biopsy” and early non-invasive markers of developing kidney disease.
Link to the paper:
Genome-wide mRNA profiling in urinary extracellular vesicles reveals stress gene signature for diabetic kidney disease
Published in:
iScience
Read more on the University of Helsinki’s webpage:
Stress markers in diabetic kidney disease exposed in urinary vesicles

BEAt-DKD symposium
Aligned with the final Consortium meeting, BEAt-DKD proudly presented research highlights and important take-home messages from 7 fruitful years of BEAt-DKD collaboration, by organising a public BEAt-DKD symposium: Successful stories, lessons learned and what’s next? on June 21,2023, in Malmö

Final BEAt-DKD annual Plenary meeting
For the last time as a consortium, BEAt-DKD scientists and members of our strategic Advisory Board met for the Annual Plenary meeting, this time at the Coordinator site at Lund University, Malmö, Sweden, on 19-20 June 2023. After almost 7 years, the project members had many exciting new data and overall great progress to present and discuss!

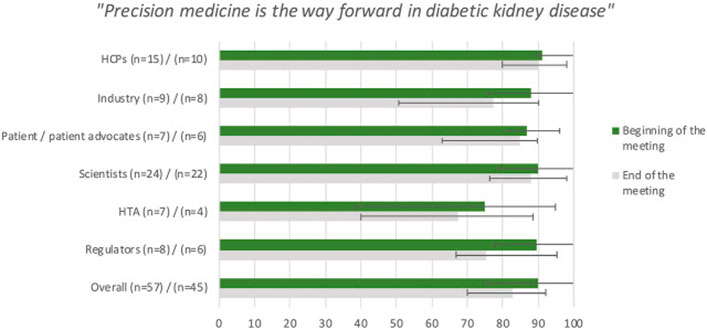
Copyright © 2021 Bakker, Mol, Nabais, Vetter, Kretzler, Nolan, Mayer, Sundgren, Heerspink, Schiel, de Vries, Gomez, Schulze, de Zeeuw and Pena.
Perspectives on a Way Forward to Implementation of Precision Medicine in Patients With Diabetic Kidney Disease; Results of a Stakeholder Consensus-Building
As part of an ongoing effort of moving implementation of precision medicine in DKD forward, a two-day consensus-building meeting was organized with different stakeholders involved in drug development and patient care in DKD, including patients, patient representatives, pharmaceutical industry, regulatory agencies representatives, health technology assessors, healthcare professionals, basic scientists, and clinical academic researchers. The study summarizes their views with regards to benefits and obstacles of implementing precision medicine in diabetic kidney disease (DKD) and discusses how to build consensus about a way forward to treat, prevent, or even reverse this disease.
Link to the paper:
https://pubmed.ncbi.nlm.nih.gov/34025424/
Published in:
Frontiers in Pharmacology

5th BEAt-DKD annual plenary meeting
Scientists of the BEAt-DKD consortium and members of our strategic Advisory Board convened for the Annual Plenary meeting at the Nicolaus Hotel in Bari, Italy on 2-4 May 2022, with additional 30 scientists participating remotely. Five years into the project, work packages presented exciting new data and overall great progress. Next Plenary meeting will be organized in June 2023, by the academic lead Lund University, in Malmö. Sweden.
iBEAt protocol published
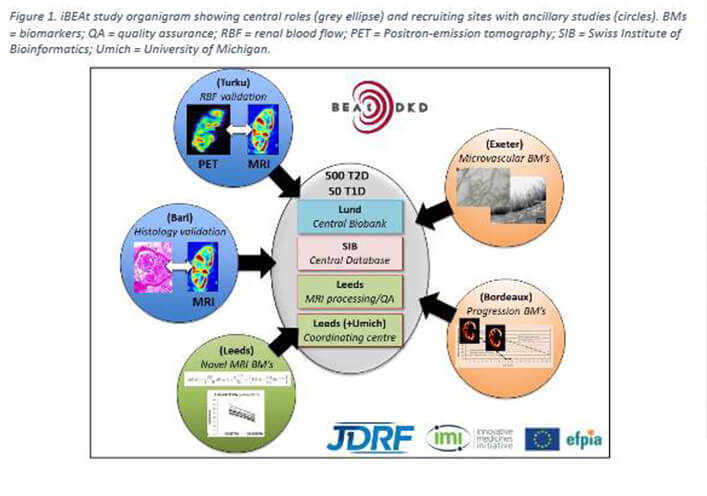
iBEAt protocol paper accepted in BMC Nephrology
iBEAt is a prospective multi-centre observational cohort study recruiting 500 patients with type 2 diabetes (T2D) and eGFR > 30ml/min/1.73m2. Being the largest DKD imaging study to date, iBEAt will provide invaluable insights into the progression and heterogeneity of DKD, and aims to contribute to a more personalized approach to the management of DKD in patients with type 2 diabetes.
https://doi.org/10.1186/s12882-020-01901-x
Control podocytes
GSK3 knockout podocytes
Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function
Preclinical studies show that the enzyme Glycogen Synthase Kinase 3 (GSK3) is a critical regulator of cellular function in podocytes and nephrocytes. Mechanistically GSK3 is important for maintaining the differentiated phenotype of these cells and preventing them from re-entering the cell cycle resulting in apoptosis through mitotic catastrophe.
www.doi.org/10.1038/s41467-018-08235-1

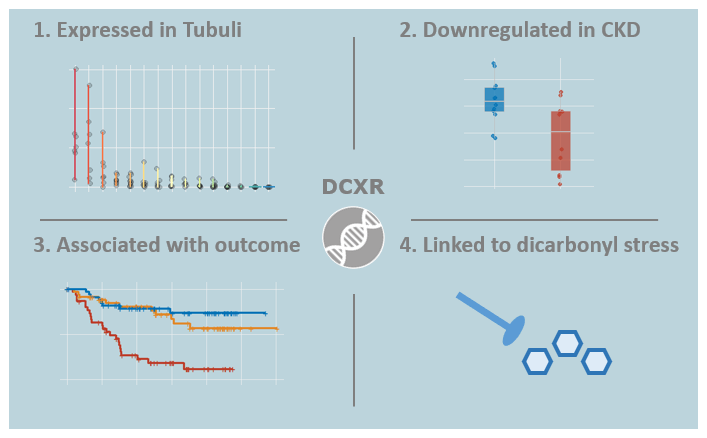
Identification of dicarbonyl and L-xylulose reductase as a therapeutic target in human chronic kidney disease
In this study it was shown for the first time that decreased expression of the renoprotective factor dicarbonyl and L-xylulose reductase (DCXR) is associated with human chronic kidney disease (CKD) progression. DCXR is mainly expressed in renal proximal tubuli and DCXR expression was also significantly reduced in CKD as compared to healthy controls. Due to its mechanistic link to the degradation of dicarbonyl compounds, DCXR depicts a novel target in CKD.
doi.org/10.1172/jci.insight.128120

2nd Symposium on Precision Medicine in Diabetic Kidney Disease – Stakeholders´Perspectives: Amsterdam, Netherlands, 3-4 April 2019
The goal of this symposium is to reach a consensus on how to move Precision Medicine into daily clinical practice of treating diabetes. We think that this will only happen if we can align stakeholder opinions and goals. Therefore, this symposium invites stakeholders from patient organizations, physician groups, drug regulators, health technology assessors, academia, and industry to participate in brainstorming and discussion. This meeting is part of WP6, the IMI2 BEAT-DKD project.
For more information on the symposium:

2nd BEAt-DKD annual plenary meeting
More than 80 scientists of the BEAt-DKD consortium and members of our strategic Advisory Board convened for the Annual Plenary meeting at the Biomedicum in Helsinki, Finland on 15-16 October 2018. Two years into the project, work packages presented exciting new data and overall great progress. Next Plenary meeting will be organized on 15-16 October 2019, by the academic co-lead University Medical Center Groningen, the Netherlands.
Supplement of the journal Diabetes, Obesity & Metabolism
BEAt-DKD aims to foster personalized medicine for diabetic kidney disease. Personalized pharmacotherapy requires an integrated effort of many stakeholders including healthcare providers, the academic community, the pharmaceutical industry, trial designers, health policy makers, regulatory authorities, insurance companies, doctors, patients and the general public. Early engagement of these stakeholders is important as they may have different priorities. BEAt-DKD participants organized a conference on personalized medicine in diabetic kidney disease in December 2017 in Groningen, the Netherlands to discuss the state of the art, challenges and solutions for successful implementation of personalized medicine in diabetic kidney disease. A summary and main findings of this conference are now published in a dedicated supplement of the scientific journal Diabetes, Obesity & Metabolism.
Diabetes, Obesity & Metabolism, Volume 20, Issue S3.
Guest editor: Hiddo J. L. Heerspink

First BEAt-DKD Plenary meeting held in October 2017
One year after the start of BEAt-DKD on 1st September 2016, more than 60 scientists of the BEAt-DKD consortium convened for the first Plenum meeting in a pleasant and very positive atmosphere in Oberursel, Germany on 5-6 October 2017. During the assembly, kindly hosted by project co-leader Sanofi, the six project work packages presented already great progress and achievements for these first 12 months. The Plenary meeting also gave the opportunity for many of our researchers to meet in person for the first time and to explore joint research interests, while aligning our key research activities towards project year two.
We were very happy to welcome the members of our strategic Advisory Board, advising us on research strategies and focus points, which will further improve the smooth running and the achievement of future goals of the project.
Overall, the first annual meeting of the BEAt-DKD consortium, with a near complete representation from our 31 partners, resulted in a very fruitful meeting, leaving everyone feeling happy after 2 days of intensely working together towards the final goal of BEAt-DKD: to elucidate targetable mechanisms and pathways underlying the initiation and progression of DKD.
The second Plenary meeting will be organized by the academic co-lead University of Helsinki on 15-16 October 2018.

Boehringer-Ingelheim joins the BEAt-DKD consortium as new participant
As 7th EFPIA partner, Boehringer-Ingelheim has joined BEAt-DKD on 13 Nov 2017 to bring their expertise in research on diabetic kidney disease (DKD) into the consortium. Their comprehensive knowledge in research and development of compounds for the treatment of diabetes and cardio-vascular diseases will support the overall project goal to elucidate targetable mechanisms and pathways underlying the initiation and progression of DKD. Analytical assays and pre-clinical models provided by Boehringer-Ingelheim will complement technologies already available in the consortium for the identification and validation of biomarkers of disease progression and treatment response as a first step towards precision medicine of DKD.